Introduction
At the primary school level, you learnt about science as a general subject. In secondary school, you will build upon this knowledge in three branches of science. These are biology, chemistry and physics.
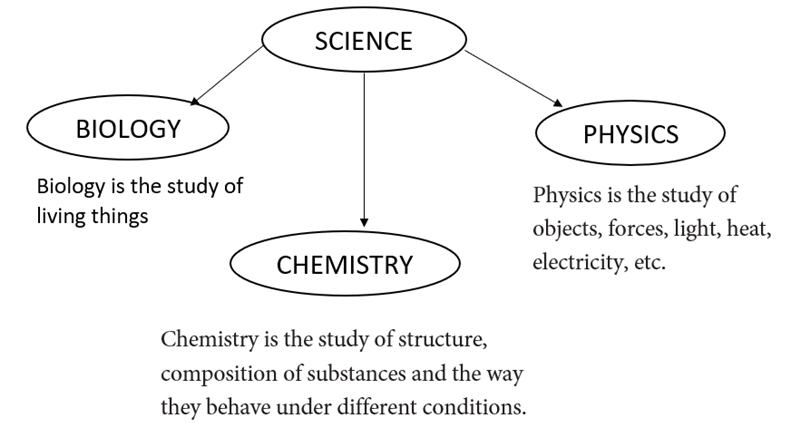
The three branches of science above can be applied in other subject areas, such as agriculture and home science.
Review of chemistry topics covered at the primary school level
Primary school covered the basics of these aspects of chemistry:
- Matter and its properties
- Mixtures and their separations
- Conductors and non-conductors
- Drugs and drug abuse
Matter and its properties
In primary school science, you learnt about matter. You learnt that matter is anything that occupies space and has mass. The heavier the object is, the more mass it has. You also learnt that matter exists in three different states, namely:
- Solids– e.g. stone, metal, and wood
- Liquids– e.g. water, kerosene, and petrol
- Gases– e.g. air
In chemistry, you will study how matter behaves, and how the state of a substance can be changed under certain conditions. For example, liquids and solids cannot be compressed, and thus their volumes cannot be changed by changing the pressure.
Properties of the three states of matter
The three states of matter have the following properties:
- Solids have a definite shape, and a fixed volume.
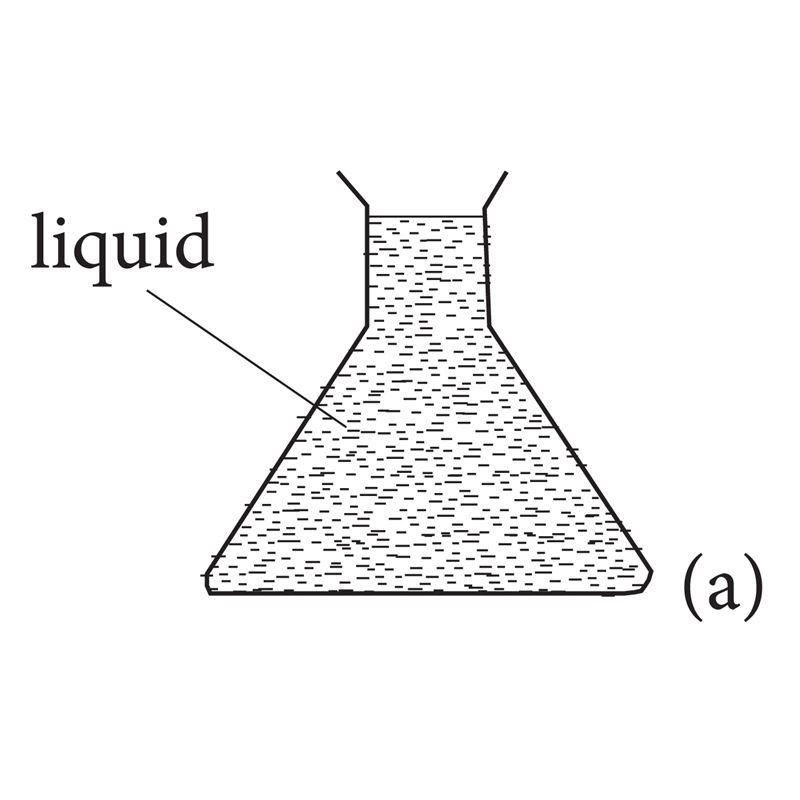
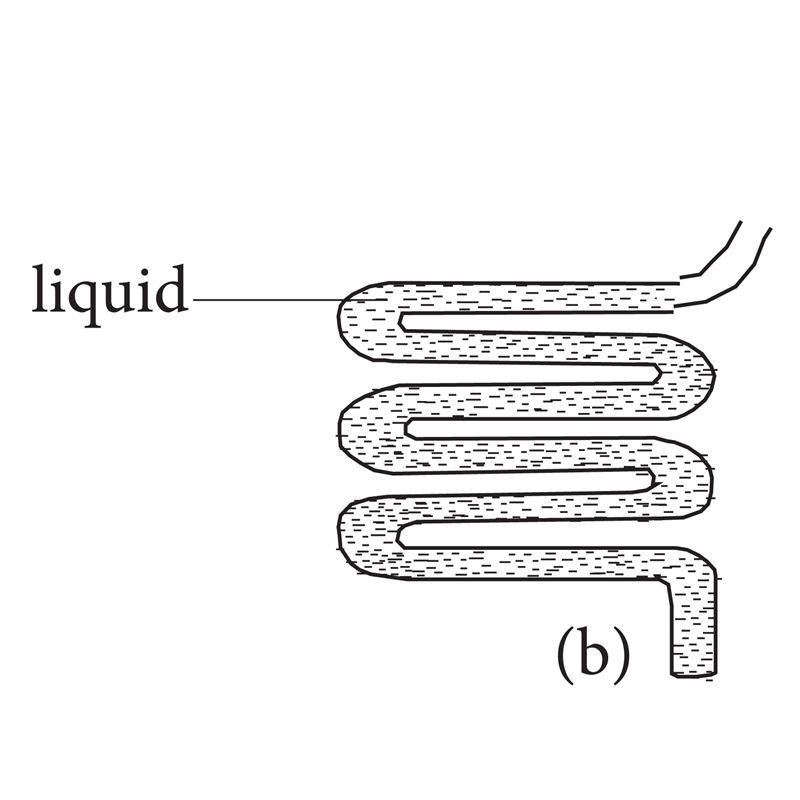
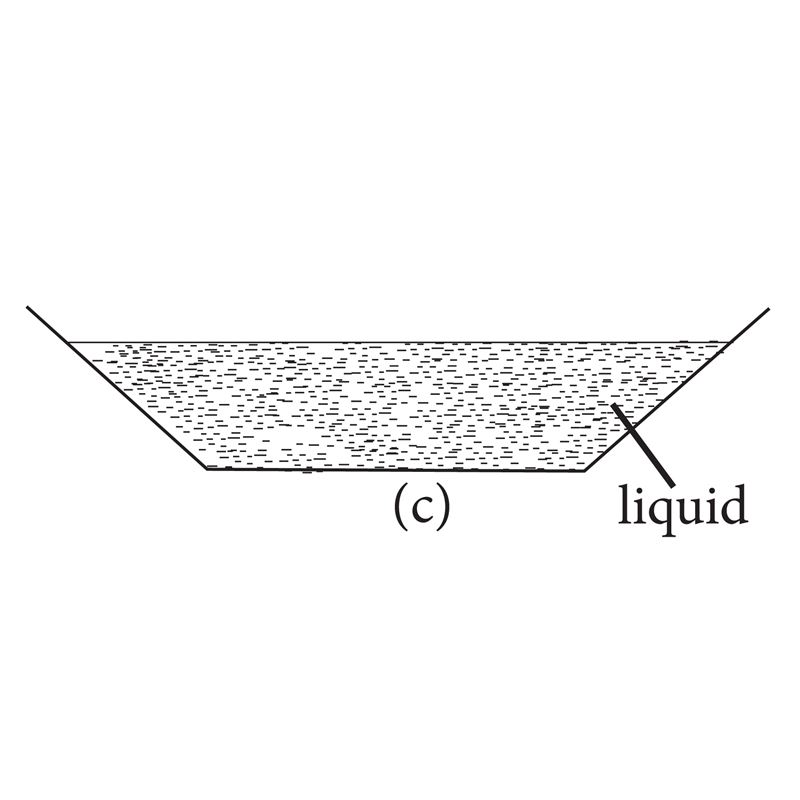
- Liquids have a fixed volume, but no definite shape. Instead, they take up the shape of the container they are in.
- Gases have no definite shape or volume. They take up all the space of their container.
Fig 1.2: Liquids can take the shape of their container
The following table gives a summary of the properties of the three states of matter:
Table: 1.1: Summarised differences of the states of matter
Solid | Liquid | Gas | |
i) Shape | Definite shape | No definite shape | No definite shape |
ii) Volume | Fixed volume | Fixed volume | No fixed volume |
iii) Density | Very high | High | Low |
iv) Flow | Does not flow | Flows easily down a slope | Flows easily in all directions |
v) Packing of particles | Tightly packed | Close together | Far apart |
The three types of matter will also respond to certain changes. For example, when the temperature is increased, they all expand, increasing in volume. The effect is much greater for a gas, which will have a much bigger volume, than for a solid or liquid. You will learn more about matter in Unit 2.
Apparatus and chemicals
A beaker, a boiling tube, water, sand, and a balloon.
Procedure
- Fill the beaker or boiling tube with water so that the water level is even with the rim, then add a bit more water. What happens?
- Fill the beaker with sand so that the sand is level with the rim, then keep adding sand. What happens?
- Inflate a balloon by blowing into it. What happens when you continue blowing after it is fully inflated? Why is this the case?
Liquids and solids visibly fill a container. Once the container is completely full, adding more will cause it to spill over.
Remember, a container is not as empty as it appears. Before a liquid or solid is added to the container, it is usually full of air that we cannot see. The liquid or solid displaces the air in the container when it is added.
Air occupies the space inside the balloon while it is being inflated. It bursts when you continue blowing because the balloon is stretched by the pressure of the air inside it. These experiments show that liquids, solids, and gases occupy space.
Apparatus and chemicals
Two empty tins, sand, and water.
Procedure
- Lift the 'empty' tin.
- Fill the other tin with sand or water and lift it using your other hand. What do you feel?

- Compare the two masses.
You will realise that the tin full of air is lighter than the tin full of other substances like sand or water.
You can therefore conclude that all matter has mass, but some matter (gas) has less mass compared with others (liquid or solid).
Mixtures and their methods of separation
You may have separated some mixtures at home or in school recently. Imagine you were tasked with separating a mixture of maize and beans. Which method would you use to achieve this?
You may also have to separate sand from water. How would you do this? How can you get clean water from muddy water?
Maize and beans can be separated by hand-picking.
Sand can be separated from water by pouring the water out of the container. This method is called decantation.
Clean water can be obtained from muddy water by running it through filter paper or cloth in a funnel. This method is called filtration.
Conductors and non-conductors
Apparatus and chemicals
A dry cell, a lightbulb, connecting wires, iron, wood, copper, lead, charcoal, aluminium, paper, zinc, sulphur, and graphite.
Procedure
- Arrange the apparatus as shown in Figure 1.4.
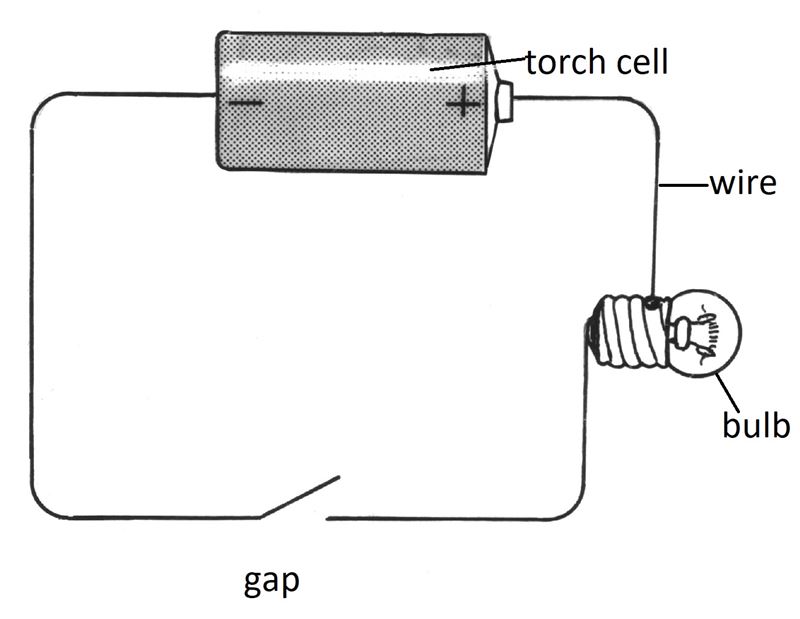
- Observe what happens when you use the various metals and non-metals listed above to close the gap.
- Sort the solids used to close the gap into those which allow the bulb to light up, and those that do not. Complete the table.
Allow the bulb to light up | Do not allow the bulb to light up |
You will find that some solids allow the bulb to light up, while others do not. This means that only some solids allow electricity to pass through them. The solids that allow electricity to pass through them are known as conductors. Those that don't are called non-conductors. All metals and graphite are conductors. All non-metals, except graphite, are non-conductors.
Drugs and drug abuse
A drug is any natural or human-made substance that changes the way in which our bodies work. For example, most medicines are drugs. Medicines can be prescribed, or provided 'over the counter'.
Over-the-counter (OTC) drugs
These are drugs which can be bought from a pharmacy or other shops without a doctor’s prescription. The most common OTC drugs include painkillers (such as paracetamol), anti-acids, and a variety of cough syrups.
Prescription drugs
Other medicines can only be obtained with a prescription from a qualified doctor or health worker. These drugs need to be taken according to the doctor's instructions. It is important to avoid overdosing or underdosing on these drugs, as this can lead to serious health problems.
Drug abuse
Drug abuse is the use of a drug for something other than its intended purpose. Over-the-counter drugs such as painkillers, anti-acids, headache tablets, malaria tablets, and sleeping pills can easily be abused. Always be careful when using these drugs.
Other commonly abused drugs, which are taken for various reasons, include alcohol, tobacco, bhang, miraa (khat), heroin, cocaine, shisha, LSD, morphine, Mandrax, and many others. These drugs are often used to give a false feeling of well-being. They have bad side effects, and can be very damaging to one’s health, or even be lethal. This is why it is illegal in this country to possess, use, or sell illegal drugs.
Drug dependence
Drug dependency comes from abusing substances that are addictive, making the user physically uncomfortable when they do not use them. These include alcohol, tobacco, bhang, miraa (khat), heroin, cocaine, and many others.
Prevention of drug abuse
You can prevent drug abuse by:
- following instructions for taking medicinal drugs.
- never taking illegal drugs.
- keeping away from drug sellers and users that encourage it.
- educating yourself about mental health, and taking care of yourself in a safe and healthy way. This will help you live a fulfilling life where drug abuse isn't a last resort.
- ask for help from your support group or professionals when you feel the need to abuse drugs.