The names and symbols of the first twenty elements of the periodic table
In Form I, we learnt that there are about 119 known elements. We have also learnt the names and symbols for some of the common elements, and how they can be arranged according to their behaviour under different conditions.
In this class, we shall learn how to arrange more elements according to their behaviour, and practise the use of more chemical symbols, particularly those of the first 20 elements of the periodic table. These are listed in Table 1.0 below.
Table 1.0: The names and symbols of the first twenty elements of the Periodic Table
Name of element | Chemical symbol | |
1 | Hydrogen | H |
2 | Helium | He |
3 | Lithium | Li |
4 | Beryllium | Be |
5 | Boron | B |
6 | Carbon | C |
7 | Nitrogen | N |
8 | Oxygen | O |
9 | Fluorine | F |
10 | Neon | Ne |
11 | Sodium | Na |
12 | Magnesium | Mg |
13 | Aluminium | Al |
14 | Silicon | Si |
15 | Phosphorus | P |
16 | Sulphur | S |
17 | Chlorine | Cl |
18 | Argon | Ar |
19 | Potassium | K |
20 | Calcium | Ca |
Drag the chemical symbols to the correct names.
- K
- Cl
- Ca
- P
- Potassium
- Chlorine
- Calcium
- Phosphorus
Components of the atom
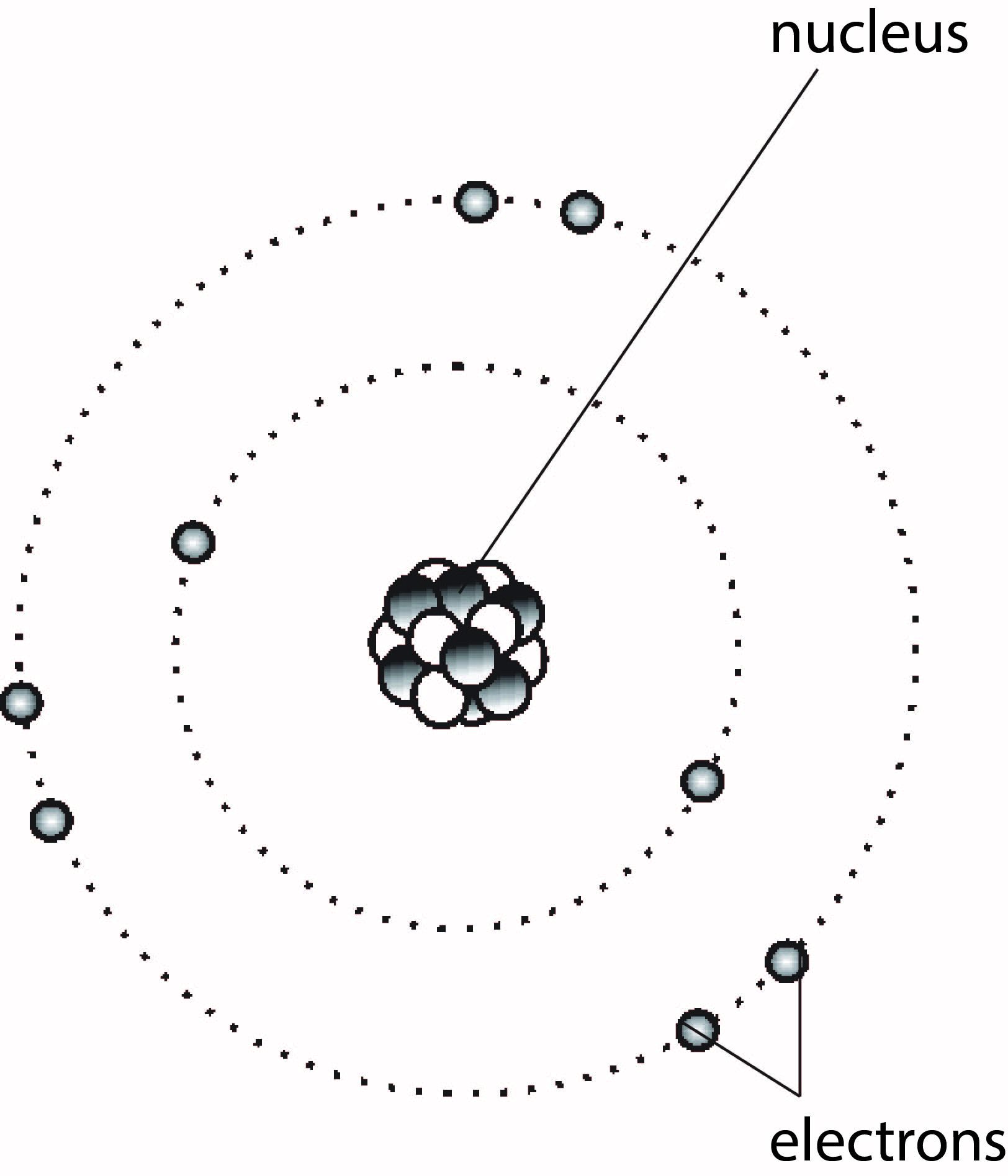
Atoms are very small particles of an element, but they contain even smaller particles that are sub-atomic. There are three kinds of sub-atomic particles: protons, electrons, and neutrons. The protons and neutrons are found in the nucleus, which is the middle part of the atom.
The nucleus is very dense and extremely small. Outside the nucleus is a much larger region of the atom where electrons occur. The electrons circulate the nucleus at different distances depending on their 'energy levels'. The simple structure of an atom is represented in Figure 1.1.
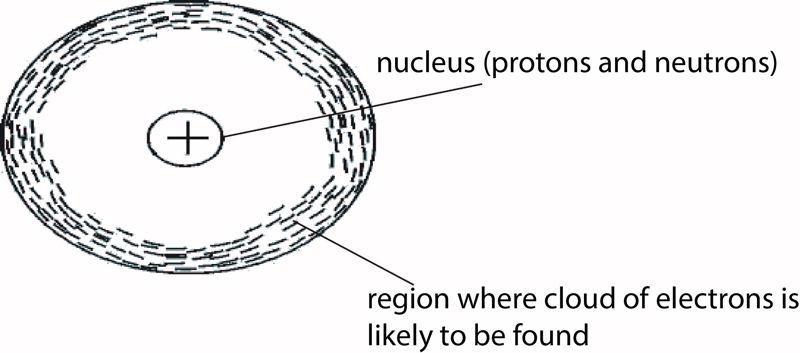
Electrons are negatively charged (–), protons are positively charged (+), and neutrons have no charge. The nucleus is made up of protons and neutrons, so the nucleus has a positive charge, as illustrated in Figure 1.1.
If we magnify the nucleus, we can see the sub-atomic particles (protons and neutrons) as illustrated in Figure 1.2.
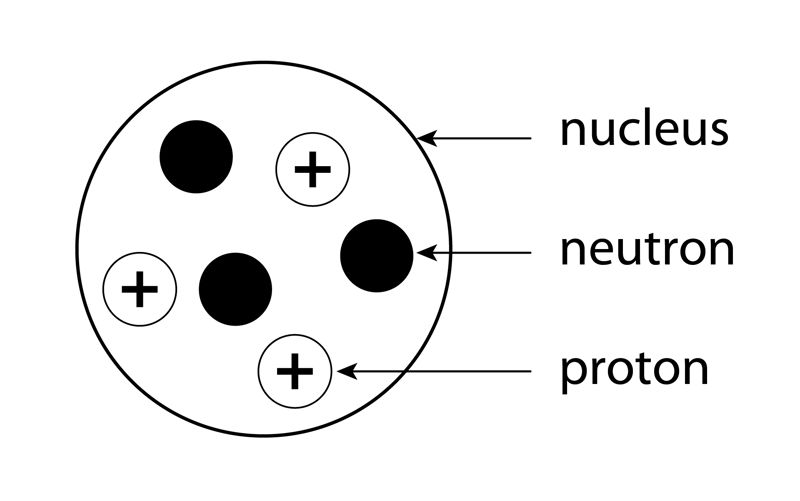
Protons repel each other due to their similar positive charge (+), but the repulsion is minimised by the presence of the neutrons, as illustrated in Figure 1.3.
The nucleus
- in the centre of the atom.
- contains protons and neutrons.
- has a positive charge because of the protons.
- the whole mass of the atom is concentrated in the nucleus.
- it is tiny compared to the overall size of the atom.
The electrons
- move around the nucleus.
- are negatively charged.
- are tiny, but cover a lot of space.
- its energy level determines its distance from the nucleus.
In a neutral atom:
Number of protons = Number of electrons
This means that each proton (+) has an electron (–) as a partner.
Table 1.1 The properties of an atom
Sub-atomic particle | Mass | Charge | Location in the atom |
Proton | 1 | + (positive) | inside nucleus |
Neutron | 1 | 0 (neutral) | inside nucleus |
Electron | – (negative) | around nucleus |
Select the correct descriptions for the components of an atom.
- In a neutral atom, the number of protons is equal to the number of neutrons.
- The electrons move around the nucleus.
- The whole mass of the atom is concentrated in the nucleus.
- The nucleus contains both protons and neutrons.
- The nucleus has a positive charge because of the neutrons.
The structure of the atom
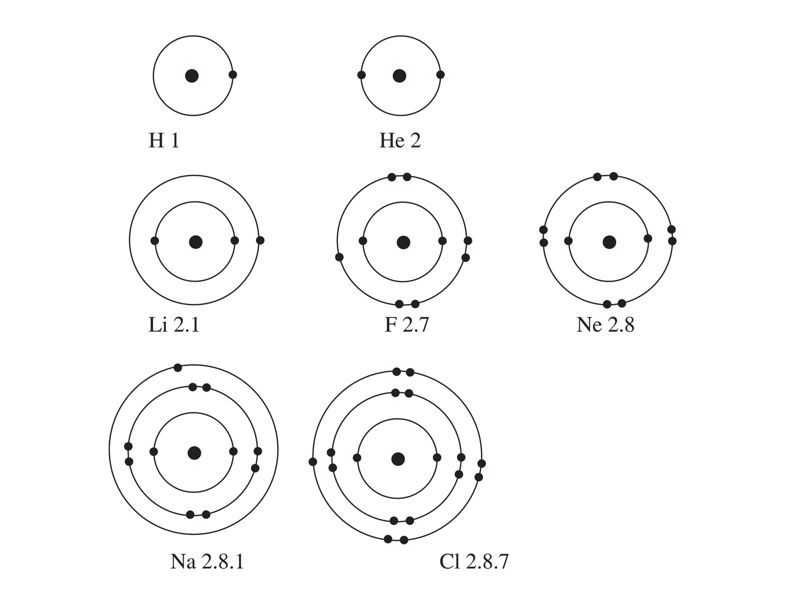
The energy levels of an atom are represented by a series of equidistant circles sharing the same centre (the nucleus). The electrons are usually represented by dots (•) or crosses (x) on these circles. The energy levels are labelled 1st, 2nd, 3rd, 4th, and so on, starting from the one nearest to the nucleus, as shown in Figure 1.4.
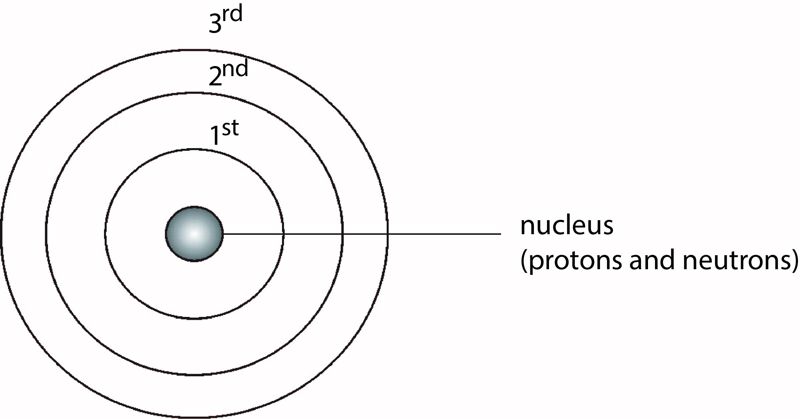
Electrons in the 1st energy level have less energy than those in the 2nd energy level, which have less energy than those in the 3rd energy level, and so on.
The 1st energy level usually has a maximum of two electrons, while the 2nd energy level has a maximum of eight electrons. The maximum number of electrons that an energy level can hold is given by the formula 2n2, where n = the number of the energy level. This means that when we draw the atomic structure, we should never put more than two electrons in the 1st energy level, or more than eight in the 2nd level.
The third energy level is usually not full, but can accommodate up to eight electrons for the first 20 elements. The first twenty elements of the periodic table can have up to 20 protons and 20 electrons. The number of electrons in each energy level is summarised in Table 1.2.
Table 1.2: The electron arrangements of the first twenty elements in the periodic table
Element | Symbol | Number of protons | 1st Energy level | 2nd Energy level |
Hydrogen | H | 1 | ・ | |
Helium | He | 2 | ・・ | |
Lithium | Li | 3 | ・・ | ・ |
Beryllium | Be | 4 | ・・ | ・・ |
Boron | B | 5 | ・・ | ・・・ |
Carbon | C | 6 | ・・ | ・・・・ |
Nitrogen | N | 7 | ・・ | ・・・・・ |
Oxygen | O | 8 | ・・ | ・・・・・・ |
Fluorine | F | 9 | ・・ | ・・・・・・・ |
Neon | Ne | 10 | ・・ | ・・・・・・・・ |
Sodium | Na | 11 | ・・ | ・・・・・・・・ |
Magnesium | Mg | 12 | ・・ | ・・・・・・・・ |
Aluminium | Al | 13 | ・・ | ・・・・・・・・ |
Silicon | Si | 14 | ・・ | ・・・・・・・・ |
Phosphorous | P | 15 | ・・ | ・・・・・・・・ |
Sulphur | S | 16 | ・・ | ・・・・・・・・ |
Chlorine | Cl | 17 | ・・ | ・・・・・・・・ |
Argon | Ar | 18 | ・・ | ・・・・・・・・ |
Potassium | K | 19 | ・・ | ・・・・・・・・ |
Calcium | Ca | 20 | ・・ | ・・・・・・・・ |
Element | Symbol | 3rd Energy level | 4th Energy level | Total electron arrangement |
Hydrogen | H | 1 | ||
Helium | He | 2 | ||
Lithium | Li | 2.1 | ||
Beryllium | Be | 2.2 | ||
Boron | B | 2.3 | ||
Carbon | C | 2.4 | ||
Nitrogen | N | 2.5 | ||
Oxygen | O | 2.6 | ||
Fluorine | F | 2.7 | ||
Neon | Ne | 2.8 | ||
Sodium | Na | ・ | 2.8.1 | |
Magnesium | Mg | ・・ | 2.8.2 | |
Aluminium | Al | ・・・ | 2.8.3 | |
Silicon | Si | ・・・・ | 2.8.4 | |
Phosphorous | P | ・・・・・ | 2.8.5 | |
Sulphur | S | ・・・・・・ | 2.8.6 | |
Chlorine | Cl | ・・・・・・・ | 2.8.7 | |
Argon | Ar | ・・・・・・・・ | 2.8.8 | |
Potassium | K | ・・・・・・・・ | ・ | 2.8.8.1 |
Calcium | Ca | ・・・・・・・・ | ・・ | 2.8.8.2 |
Studying the arrangement of electrons in an atom helps us to explain the patterns in the properties of the elements. These properties are the basis for the periodic table, which we will discuss later. For now, let us observe the electron arrangement of the following atoms in Figure 1.5: Hydrogen, helium, lithium, fluorine, neon, sodium, and chlorine.
Investigate the structure of the atom and select the correct electron arrangement to describe it.
- 2.8.7
- 2.8.8
- 2.8
- 2.7
